
by Chris Woodford. Last updated: May 17, 2023.
Nature has given ussome amazing materials. There's wood: a material so strong andversatile you can use it for everything from making paper to buildinghouses. There's also wool, with insulation so effective it lets sheepstand outside in the snow all winter. Or how about skin: a materialthat will repair itself automatically and often completely invisiblyin only a matter of days? Truly incredible though these materialsare, they're far from perfect for every application,especially in the modern world where the challenges we face are onesnature could never have anticipated. That's why we now rely onsynthetic materials such as Kevlar®. It's a plastic strong enough tostop bullets and knives—often described as being "five timesstronger than steel on an equal weight basis." [1] It has many other uses too, from making boats and bowstrings to reinforcing tires and brake pads. [2]Let's take a closer look at how it's made and what makesit so tough!
Photo: Kevlar is best known as a protective material, but it's muchmore versatile than that. This is a piece of woven Kevlar being used as partof a proposed, inflatable "space tent" for use on the Moon or Mars.Photo by Paul Hudson published on Wikimedia Commons under a Creative Commons (CC BY 2.0) licence.
Sponsored links
Contents
- What exactly is Kevlar?
- What's so good about Kevlar?
- How is Kevlar made?
- What's Kevlar used for?
- What makes Kevlar such a good antiballistic material?
- Find out more
What exactly is Kevlar?
Kevlar is one of those magic modern materials people talk about all the time without everreally explaining any further. "It's made of Kevlar", they say,with a knowing nod, as though that were all the explanation youneeded.
Kevlar is simply a super-strong plastic. If that sounds unimpressive, remember that there are plastics—and there are plastics.There are literally hundreds of synthetic plastics made bypolymerization (joining together long chain molecules) and they havewidely different properties. Kevlar's amazing properties are partly due to its internal structure (how its molecules are naturally arranged in regular, parallel lines) and partly due to the way it's made into fibers that are knitted tightly together.[3]

Photo: Kevlar textiles get their properties partly from the inherent strengthof the polymer from which the fibers are made and partly from the way the fibers are knitted tightly together, as shown herein a NASA ballistics test. Picture courtesy of NASA Glenn Research Center (NASA-GRC) and Internet Archive.
Kevlar is not like cotton—it's not something anyone can make from the right rawmaterials. It's a proprietary material made only by the DuPont™chemical company and it comes in two main varieties called Kevlar 29and Kevlar 49 (other varieties are made for special applications). [4]In its chemical structure, it's very similar to another versatileprotective material called Nomex. Kevlar and Nomex are examples ofchemicals called synthetic aromatic polyamides or aramids for short.Calling Kevlar a synthetic aromatic polyamide polymer makes it sound unnecessarily complex.Things start to make more sense if you consider that description one word at a time:
- Synthetic materials are made in a chemical laboratory(unlike natural textiles such as cotton, which grows on plants, andwool, which comes from animals).
- Aromatic means Kevlar's molecules have a strong, ring-likestructure like that of benzene.
- Polyamide means the ring-like aromatic molecules connecttogether to form long chains. These run inside (and parallel to) thefibers of Kevlar a bitlike the steel bars ("rebar") in reinforced concrete.
- Polymer means that Kevlar is made from many identicalmolecules bonded together (each one of which is called a monomer). Plastics are the most familiar polymersin our world. As we've seen,the monomers in Kevlar are based on a modified, benzene-like ringstructure.
Like Nomex, Kevlar is a distant relative of nylon, the first commercially successful"superpolyamide", developed by DuPont in the 1930s. Kevlar was introduced in 1971,having been discovered in the early 1960s by US chemist Stephanie Kwolek (1923–2014), who earned US Patent 3,287,323 for her invention, with Paul Morgan, in 1966. Originally developed as a lightweight replacement for steel bracing in vehicle tires,it's probably best-known today for its use in things like body armor; by the time of Kwolek's death in 2014, one million Kevlar body vests had been sold—and countless lives saved.[5]
What's so good about Kevlar?

Photo: Braided Kevlar can be used to make super-strong rope. Compared on a strength-to-weight ratio, Kevlar is about 5–6 times stronger than steel wire and twice as strongas ordinary nylon fiber. Picture by Casey H. Kyhl courtesy of US Navy andWikimedia Commons.
These are some of Kevlar's properties:
- It's strong but relatively light. The specific tensilestrength (stretching or pulling strength) of both Kevlar 29 and Kevlar 49 is over eight times greaterthan that of steel wire.[6]
- Unlike most plastics it does not melt: it'sreasonably good at withstanding temperatures and decomposes only atabout 450°C (850°F).
- Unlike its sister material, Nomex, Kevlar can be ignited but burningusually stops when the heat source is removed.
- Very low temperatureshave no effect on Kevlar: DuPont found "no embrittlement ordegradation" down to −196°C (−320°F).
- Like other plastics, longexposure to ultraviolet light (in sunlight, for example) causesdiscoloration and some degradation of the fibers in Kevlar.
- Kevlar can resist attacks from many different chemicals, though long exposure to strongacids or bases will degrade it over time.
- In DuPont's tests, Kevlar remained "virtually unchanged" after exposure to hotwater for more than 200 days and its super-strong properties are"virtually unaffected" by moisture.
And what's bad?
It's worth noting that Kevlar also has its drawbacks. In particular, although it hasvery high tensile (pulling) strength, it has very poor compressive strength (resistance tosquashing or squeezing). That's why Kevlar isn't used instead of steel as a primarybuilding material in things like buildings,bridges, and other structures where compressive forces are common.
Sponsored links
How is Kevlar made?
There are two main stages involved in making Kevlar. First you have to produce the basicplastic from which Kevlar is made (a chemical called poly-para-phenyleneterephthalamide—no wonder they call it Kevlar). Second, you have toturn it into strong fibers. So the first step is all about chemistry;the second one is about turning your chemical product into a moreuseful, practical material.
Polyamides like Kevlar are polymers (huge molecules made of many identical parts joinedtogether in long chains) made by repeating amides over and overagain. Amides are simply chemical compounds in which part of anorganic (carbon-based) acid replaces one of the hydrogen atoms inammonia (NH3). So the basic way of making a polyamide is to take anammonia-like chemical and react it with an organic acid. This is anexample of what chemists call a condensation reaction because two substances fusetogether into one.[7]
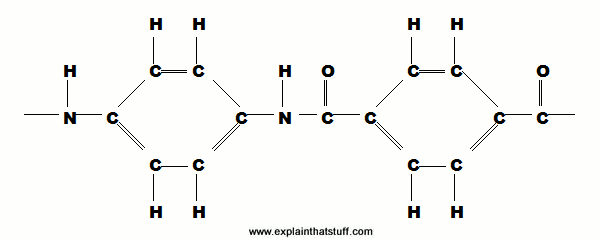
Artwork: Kevlar's monomer: C=carbon, H=hydrogen, O=oyxgen, N=nitrogen, — is a single chemical bond, and = is a double bond. This basic building block is repeated over and over again in the very long chains that make up the Kevlar polymer. Source: "US Patent: 3287323: Process for the production of a highly orientable, crystallizable, filament" by Stephanie Kwolek et al.
Kevlar's chemical structure naturally makes it form in tiny straight rods that packclosely together, like lots of stiff new pencils stuffed tightly intoa box (only without the box). These rods form extra bonds between one another (known as hydrogen bonds)giving extra strength—as though you'd glued the pencils together as well. This bonded rod structure is essentially what gives Kevlar its amazing properties.(More technically speaking, we can say the Kevlar rods are showing what's callednematic behavior (lining up in the same direction), which is also what happens in the liquid crystals used in LCDs (liquid crystal displays).)
You probably know that natural materials such as wool and cotton have to be spun into fibersbefore they can turned into useful textile products—and the same istrue of artificial fibers such as nylon, Kevlar, and Nomex. The basic aramid is turned into fibers by a process called wet spinning, which involves forcing a hot, concentrated, andvery viscous solution of poly-para-phenylene terephthalamide through a spinneret (a metal former a bit like a sieve) to make long, thin,strong, and stiff fibers that are wound onto drums. The fibers are then cut to length and woven into a tough mat to make the super-strong, super-stiff finished material we know as Kevlar.[8]

Artwork: How Kevlar is made. 1) The rodlike Kevlar molecules start off in dilute solution. 2) Increasing the concentration increases the number of molecules but doesn't make them align. At this stage, the molecules are still tangled up and not extended into straight, parallel chains. 3) The wet-spinning process causes the rods to straighten out fully and align so they're all oriented in the same direction—forming what's called a nematic structure—and this is what gives Kevlar its exceptionally high strength. Image based on an original artwork from DuPont's Kevlar Technical Guide (see references below).
What's Kevlar used for?
Kevlar can be used by itself or as part of a composite material (one material combined withothers) to give added strength. It's probably best known forits use in bulletproof vests and knifeproof body armor, but it hasdozens of other applications as well. It's used as reinforcement incar tires, in car brakes, in the strings of archerybows, and in car, boat, and even aircraft bodies. It's even used in buildingsand structures, although not (because of its relatively low compressive strength) as the primary structural material.[9]

Photo: Super-strong Kevlar is best known for its use in body armor—and thisphoto shows you why: it's a piece of Kevlar after being hit by a projectile. You can see a dent (coming up toward the camera)—but you can't see a hole.You might be bruised by this impact (or suffer what's called a "blunt trauma" injury), but you wouldn't die.Picture courtesy of US Army.
What makes Kevlar such a good antiballistic material?

Photo: Think of Kevlar as a lightweight modern alternative to heavy, cumbersome, medieval suits of armor! Photo by Staff Sgt. Nate Hauser courtesy of US Marine Corps and Internet Archive.
If you've read our article on bullets, you'll know that theydamage things—and people—because they travel at high speeds with hugeamounts of kinetic energy. Although there's no such thing as completely "bulletproof," materials likebulletproof glass do a good job at protecting us by absorbing (soaking up)and dissipating (spreading out) the energy of a bullet.
Kevlar is an excellent antiballistic (bullet- and knife-resistant) material becauseit takes a great deal of energy to make a knife or a bullet pass through it. The tightly wovenfibers of highly oriented (lined-up) polymer molecules are extremely hard to move apart: it takes energy to separate them. A bullet(or a knife pushed hard by an attacker) has its energy "stolen" from it as it tries to fight its way through.If it does manage to penetrate the material, it's considerably slowed down and does far less damage.
Although Kevlar is stronger than steel, it's about 5.5 times less dense (the density of Kevlaris about 1.44 grams per cubic centimeter, compared to steel, which is round about 7.8–8 gramsper cubic centimeter). That means a certain volume of Kevlar will weigh 5–6 times less than the same volume of steel.Think back to medieval knights with their cumbersome suits of armor: in theory, modern Kevlar gives just as much protection—but it's light and flexible enough to wear for much longer periods.
More layers = more protection
If you think of Kevlar "soaking up" the energy of a bullet, it's fairly obviousthat a greater thickness of Kevlar—more layers of the material bondedtogether—will give more protection.
How much Kevlar do you need to stop a bullet? It depends on the Kevlar and it depends on the weight, type, and speed of the bullet. Kevlar comes in different weights—and bullets also come in different types and weights and travel at very different speeds, with different amounts of energy. The bigger the bullet and the faster it's travelling, the more kinetic energyit has, the further it will penetrate, and the more damage it will do. You need more layers of Kevlar to stopbigger, faster bullets than smaller, slower ones. Typically, bulletproof vests have at least 8–16 layers of Kevlar and often 32–48 layers or even more. Some vests combine Kevlar with other materials, while others use different materialsinstead of Kevlar, such as Spectra®.[10]

Chart: You need a greater thickness of Kevlar body armor to stop higher-speed (velocity) bullets. In theory, the thicker the Kevlar, the shorter the distance a bullet should be able to pass through (the shorter the penetration depth); in practice, it's a little bit more complicated than that.
Generally speaking, the more layers of heavier Kevlar you have, the more protective your "bulletproof" armor, but the heavier,bulkier, and hotter it will be to wear, and the more it will restrict your movement. You could cover yourself with a million layers of Kevlar, which might stop most everyday bullets, but it's hardly going to be practical. So there's a tradeoff to be made between protection and usability. And, where Kevlar's concerned, it's not always a matter of "thicker equals better": there's another qualification too. Bullets travel fast—a rifle bullet can be going 10 times faster than a race car—and they're designed to deform when they hit things so they do more damage. According to some recent ballistics research, the Kevlar in a bulletproof vest will affect this process, sometimes making a bullet travel further into a target than if no (or less) Kevlar were used. That's why you need a lot of Kevlar in effective bulletproof vests, both to allow for how it might alter the bullet and to soak up all the bullet's energy.
Kevlar isn't always enough
If you want to protect soldiers against high-velocity rifle bullets, you're going to need much thickerarmor than if you simply want to protect police officers against handgun bullets,which have lower velocity and less kinetic energy.It's important to remember that no material is 100 percent bulletproof—and sometimes even Kevlar isn't enough.
You can see this clearly in the official US National Institute of Justice Body Armor Classification,which ranks bulletproof vests and other body protection (made of Kevlar and other materials)on a scale from I to IV for its ability to protect against bullets fired from weapons of different power. At the low end of the scale,type IIA armor has to protect against smaller handgun bullets (typically 9mm full metal jacketedbullets weighing 8.0g or 0.3 oz and fired at about 373 m/s or 834 mph); you need at least 16 layers of Kevlar for that.Higher up the scale, type IIIA armor has to resist more powerful handheld bullets(such as .44 Magnum bullets weighing 15.6 g or 0.6 oz and fired at 436 m/s or 975 mph); that needs twice as much Kelvar—at least 30 layers. It's important to note that even Kevlar has its limits. For protection against rifle bullets (ordinary ones or armor-piercing ones), which travel much faster (850–900 m/s or 1900–2000 mph) with considerably higher kinetic energy, Kevlar isn't enough: you need body armor made from steel or ceramic plates (classified as type III and IV).
Sponsored links
Please support our site
If you found this page helpful, please
Find out more
On this website
On other sites
- Kevlar Technical Guide: Definitive information from DuPont. This will take you to a 4MB PDF format version of the Technical Guide, which can take a while to download depending on your Internet connection.
- Kevlar's uses and applications: DuPont run through about 20 different common uses of Kevlar, from adhesives to vehicle armor.
- US Patent: 3287323: Process for the production of a highly orientable, crystallizable, filament: This is Stephanie Kwolek and Paul Morgan's original Kevlar patent (filed April 25, 1963 and granted November 22, 1966), which describes various chemical methods for making Kevlar.
Books
For older readers
- Enough for One Lifetime: Wallace Carothers, Inventor of Nylon by Matthew Hermes. Chemical Heritage Foundation, 1996. A dry but nonetheless interesting biography of the inventor of nylon (a material very similar to Kevlar). It gives a good sense of how modern, revolutionary materials tend to be "corporate inventions" rather than the work of single inventors, and how inventors can completely fail to foresee the consequences of their ideas.
For younger readers
- Stephanie Kwolek: Creator of Kevlar by Gail B. Stewart. Greenhaven, 2008. A short, 48-page biography for ages 9–12.
- Girls Think of Everything: Stories of Ingenious Inventions by Women by Catherine Thimmesh. Houghton Mifflin Harcourt, 2000/2018. An inspiring read for young teenagers, keen on STEM. Stephanie Kwolek is covered in a short chapter beginning on page 28.
Articles
Popular science and news
- Kevlar 'Wallpaper' Could Protect Soldiers From RPG Blasts by Jordan Golson. Wired, May 29, 2015. How sheets of Kevlar "wallpaper" could be used to protect buildings and structures.
- Stephanie L. Kwolek, Inventor of Kevlar, Is Dead at 90 by Jeremy Pearce. The New York Times, June 20, 2014. A shortr obituary of the chemical pioneer.
- Armor on the Field: The NFL's Headlong Race to Build the Unbreakable Linebacker by Sean Conboy. Wired, July 9, 2012. Could Kevlar padding help to protect sports competitors from serious injury?
- DuPont wins $900m Kevlar spy case: The Guardian, 15 September 2011. Kevlar is an extremely valuable invention and, like other technological pioneers, DuPont has to fight big legal cases to protect its "intellectual property."
- School uniforms made slash-proof: BBC News, 14 August 2007. Could Kevlar protect children against knife crime?
- Tribute to Inventive Women: BBC News, 27 November 2003. A short article about women inventors, including Stephanie Kwolek.
- Kevlar Enters Spotlight As New 'Miracle Fiber' by Gerd Wilcke. The New York Times, May 13, 1974. How the Times described the early development of Kevlar back in the 1970s.
Technical
- Experimental study of bullet-proofing capabilities of Kevlar, of different weights and number of layers, with 9 mm projectiles by Riaan Stopforth and Sarp Adali. Defence Technology, Volume 15, Issue 2, April 2019, Pages 186–192. It's not always the case that more Kevlar provides greater stopping power for bullets, as this scientific study reveals.
- High-modulus, high-strength organic fibers in Concise Encyclopedia of Composite Materials by Anthony Kelly (ed). Pergamon Press, 1989. This is a great introduction to the chemistry and physical properties of Kevlar and similar materials.
References
- ↑Bear in mind that there are manydifferent types of steel, with widely differing properties, and note the part about"on an equal weight basis." DuPont's website is the original source for the claim; for example, on its page Kevlar Fibers. Many books repeat this information; see this selection from Google Books.Checking the Kevlar Technical Guide, we find the tensile strength of both Kevlar 29 and 49 is about 3600MPa,while the ultimate tensile strength of high-strength steels is more like 500–800Mpa.
- ↑Kevlar's uses and applications, DuPont.
- ↑Kevlar Technical Guide, DuPont, 2017, pp.3–4.
- ↑Kevlar Technical Guide, DuPont, 2017, p5.Other varieties include Kevlar 100 (for ropes), Kevlar 129 (for lightweight motorcycle gear), andquite a few more described in Kevlar® Fibers.
- ↑According to Kwolek's New York Times obituary, her team's task was "trying to develop a lightweight fiber that would be strong enough to replace the steel used in radial tires."
- ↑The source for all the information in this list is the Kevlar Technical Guide, DuPont, 2017.
- ↑The formation of Kevlar by condensation polymerization is described in the original Kwolek and Morgan patent: US Patent: 3287323: Process for the production of a highly orientable, crystallizable, filament.
- ↑For more on how synthetic fibers are manufactured, and how they get their strength, see "Chapter 4: Synthetic polymeric fibers" in Fibrous Materials by K. K. Chawla, Cambridge, 1998, p.73.
- ↑Kevlar's uses and applications, DuPont.
- ↑For a simple overview of the effectiveness ofdifferent amounts of Kevlar, see the entry "Body Armor" in The Encyclopedia of High-tech Crime and Crime-fighting by Michael Newton, Facts on File, 2003, p.41.
Please do NOT copy our articles onto blogs and other websites
Articles from this website are registered at the US Copyright Office. Copying or otherwise using registered works without permission, removing this or other copyright notices, and/or infringing related rights could make you liable to severe civil or criminal penalties.
Text copyright © Chris Woodford 2008, 2023. All rights reserved. Full copyright notice and terms of use.
"Nomex", "Kevlar", and "DuPont" are trademarks or registered trademarks of E. I. Du Pont de Nemours and Company.
"Spectra" is a registered trademark of Honeywell International Inc.
More to explore on our website...
↑ Back to top

